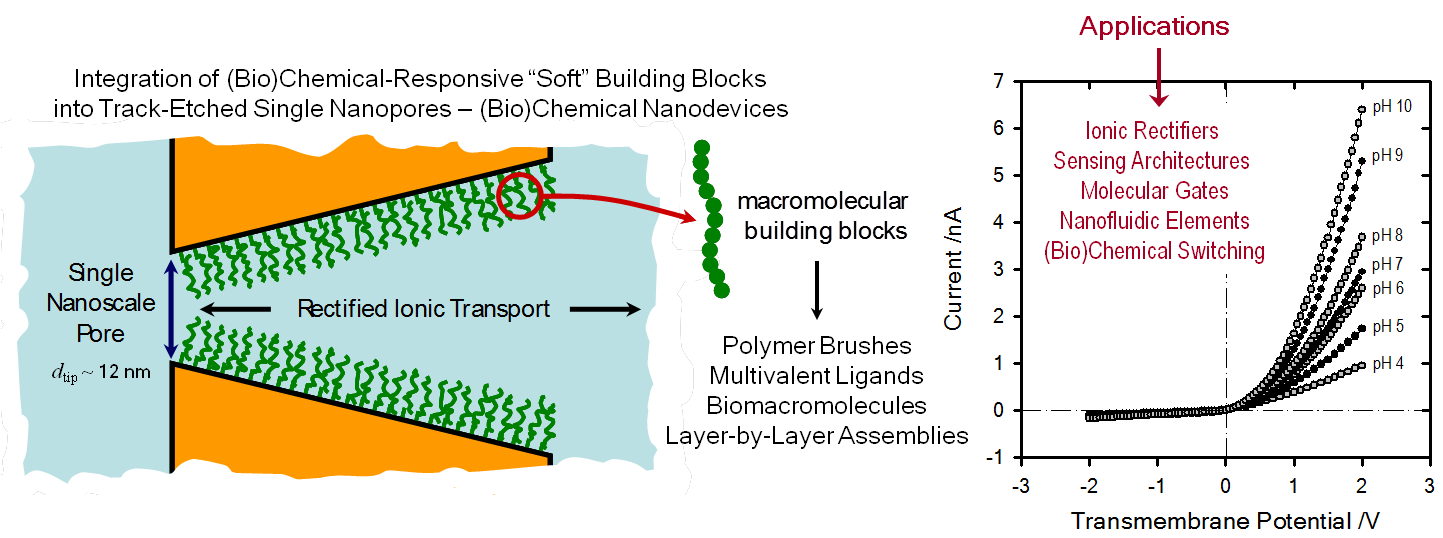
The virtues of working with nanofluidic elements are being increasingly recognized by the biomimetics research community. This has led to the emergence of a research area that is currently at the forefront of materials science and engineering. The advent of track-etching techniques (“top-down approach”) has resulted in an increasing mastery in construction of nanoscale fluidic structures and has given a decisive impetus not only to the development of this exciting area of nanotechnology but also opened up new possibilities to reproducibly engineer nanopore and nanochannel architectures with various shapes and diameters down to a few nanometers. One major attraction of these nanofluidic elements is their outstanding ability to control and manipulate the transport of chemical and biochemical species flowing through them, thus enabling the construction of ionic circuits capable of sensing, switching, or separating diverse species in aqueous solutions. Furthermore, these nanofluidic devices have also been shown to display transport properties that resemble biological protein ion channels, such as ion selectivity, current rectification, flux inhibition by protons and divalent cations, transport of ions against concentration gradients, and even ion current fluctuations. In the particular case of asymmetric nanochannels/nanopores, appealing rectification effects arise when the channel surface is charged and the dimensions are comparable to the Debye length. These fascinating physicochemical properties displayed by charged nanochannels or nanopores provided the scenario to create new functional and addressable nanofluidic architectures and also led to the birth of a whole new area of research concerning the design of nanochannel-based devices resting on surface charge governed ionic transport. However, in order to confer selectivity to solid-state nanopores, it is necessary to develop and explore new methods for functionalizing the pore walls. Hence, the creation of functional nanopores capable of acting as selective ion channels or smart nanofluidic sensors depends critically on our ability to assemble and build up molecular architectures in a predictable manner within confined geometries with dimensions comparable to the size of the building blocks themselves. In this context, we are working to integrate macro- and supramolecular assemblies into track-etched solid state nanopores in order to create chemical nanodevices displaying a wide variety of functional features. We seek to further the rich interplay between the complexity and versatility of “soft” building blocks and the remarkable physical characteristics of solid-state nanopores.